Von Willebrand Disease in US 2025
Von Willebrand Disease remains the most common inherited bleeding disorder affecting the United States population in 2025, impacting up to 1% of the general population. This genetic condition involves either a deficiency or dysfunction of von Willebrand factor, a critical protein responsible for blood clotting. While the disease affects both men and women equally in terms of genetic occurrence, women experience symptoms more frequently due to bleeding complications during menstruation, pregnancy, and childbirth. The Centers for Disease Control and Prevention, through its Community Counts surveillance program, continues to track patient demographics and treatment patterns across federally funded hemophilia treatment centers nationwide.
The current healthcare landscape for Von Willebrand Disease in the US 2025 reflects significant advances in diagnosis, treatment options, and patient management strategies. Between 2012 and 2024, more than 35,466 individuals with VWD received care at hemophilia treatment centers across the United States, representing a comprehensive patient population that provides valuable insights into disease prevalence, demographic distribution, and clinical characteristics. This data-driven approach enables healthcare providers to better understand the evolving nature of VWD management, improve treatment protocols, and address the specific needs of different patient subgroups. With enhanced diagnostic capabilities and expanded treatment options including recombinant factor therapies and prophylactic management strategies, patients diagnosed with VWD in 2025 have access to more comprehensive care than ever before.
Interesting Facts and Latest Statistics on Von Willebrand Disease in US 2025
| Key Fact Category | Statistic/Data Point | Significance |
|---|---|---|
| Overall Prevalence | Affects up to 1% of general US population | Most common inherited bleeding disorder |
| Patient Count (2012-2024) | 35,466 patients treated at HTCs | Represents tracked population in specialized care centers |
| Gender Distribution | 68% female, 32% male patients | Women more likely to seek treatment due to menstrual symptoms |
| Most Common Type | Type 1 VWD: 27,641 patients (78%) | Mildest form with partial VWF deficiency |
| Age Peak – Males | Highest prevalence at 5-14 years | Early diagnosis often through injury-related bleeding |
| Age Peak – Females | Highest prevalence at 15-24 years | Symptoms manifest during reproductive years |
| Racial Distribution | 82% White, 7% Black/African American | Reflects both genetics and healthcare access patterns |
| Type 2 VWD Cases | 3,808 patients (11%) | Qualitative defect in VWF function |
| Type 3 VWD (Severe) | 531 patients (1.5%) | Rarest and most severe form |
| Hispanic/Latino Patients | 18% of Type 1 VWD patients | Growing representation in treatment centers |
| Insurance Coverage | 98% of Type 1 patients insured | High insurance coverage facilitates treatment access |
| Pediatric Population | 43% of Type 1 patients aged 11-19 years | Significant adolescent patient population |
| Treatment Centers | Over 140 federally funded HTCs nationwide | Comprehensive specialized care network |
| Clinically Significant Cases | Approximately 1 in 1,000 require treatment | Distinction between genetic prevalence and symptomatic disease |
Data Source: Centers for Disease Control and Prevention (CDC) Community Counts Program, HTC Population Profile Patient Characteristics for Von Willebrand Disease, Data Reported from January 1, 2012 through March 31, 2024
Understanding Von Willebrand Disease statistics requires recognizing the distinction between genetic prevalence and clinically symptomatic disease. While population-based genetic studies suggest up to 1% prevalence, the actual number requiring medical intervention is considerably lower at approximately 125 per million individuals. The CDC’s Community Counts data from 2012 through March 2024 demonstrates that 35,466 patients received care specifically for VWD at hemophilia treatment centers, with the overwhelming majority diagnosed with Type 1 VWD representing 27,641 cases or 78% of all tracked patients. The data reveals significant gender disparity in treatment-seeking behavior, with 68% female patients compared to 32% male patients, primarily because women experience more noticeable bleeding symptoms during menstruation and childbirth.
The age distribution patterns provide crucial insights into disease presentation and diagnosis timing across different populations. For male patients with Von Willebrand Disease in 2025, prevalence peaks during ages 5-14 years when physical activities and sports-related injuries often lead to diagnosis. In contrast, female patients show peak prevalence during ages 15-24 years, corresponding with the onset of menstruation when heavy or prolonged menstrual bleeding becomes a primary symptom. The racial and ethnic composition of the VWD patient population shows 82% White patients, 7% Black or African American patients, with 18% identifying as Hispanic or Latino. Insurance coverage remains high across all VWD types, with 98% of Type 1 patients having insurance coverage, facilitating access to specialized hemophilia treatment centers and necessary therapies. The pediatric and adolescent population comprises a substantial portion of tracked patients, with 43% of Type 1 VWD patients falling within the 11-19 age range, highlighting the importance of early diagnosis and ongoing management throughout critical developmental years.
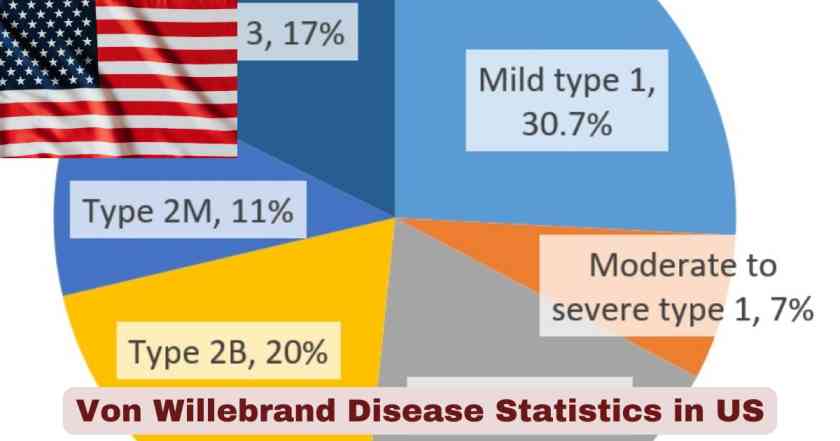
Von Willebrand Disease Type Distribution in US 2025
| VWD Type | Number of Patients | Percentage | Clinical Characteristics |
|---|---|---|---|
| Type 1 | 27,641 | 78% | Partial quantitative VWF deficiency, mild symptoms |
| Type 2 | 3,808 | 11% | Qualitative VWF defect, moderate symptoms |
| Type 3 | 531 | 1.5% | Complete VWF absence, severe bleeding |
| Type Other/Unknown | 3,486 | 9.8% | Classification pending or variant forms |
| Total | 35,466 | 100% | All VWD patients in HTC system |
Data Source: Centers for Disease Control and Prevention (CDC) Community Counts, Von Willebrand Disease Patient Characteristics, January 2012 – March 2024
The distribution of Von Willebrand Disease types across the United States demonstrates clear patterns that inform treatment approaches and resource allocation within the healthcare system. Type 1 VWD dominates the clinical landscape with 27,641 diagnosed patients representing 78% of all cases tracked through hemophilia treatment centers from 2012 through March 2024. This form involves a partial quantitative deficiency of von Willebrand factor, typically presenting with mild to moderate bleeding symptoms that may include easy bruising, frequent nosebleeds, and prolonged bleeding from minor cuts. The high prevalence of Type 1 reflects both its autosomal dominant inheritance pattern with approximately 60% penetrance and the relatively milder symptomatology that allows many patients to lead normal lives with minimal intervention.
Type 2 VWD, affecting 3,808 patients or 11% of the tracked population, represents qualitative defects in von Willebrand factor function despite potentially normal VWF levels. This category includes four distinct subtypes—2A, 2B, 2M, and 2N—each characterized by specific molecular defects affecting different aspects of VWF function. The most severe form, Type 3 VWD, affects 531 patients representing only 1.5% of cases, involving complete absence of von Willebrand factor and presenting with severe bleeding complications including spontaneous joint bleeding and life-threatening hemorrhages. The remaining 3,486 patients categorized as “Type Other/Unknown” comprise 9.8% of cases and include individuals whose VWD classification remains pending additional testing or those with variant forms not fitting standard classifications. This comprehensive categorization enables specialized hemophilia treatment centers to provide targeted therapies, with Type 1 patients often managed with desmopressin (DDAVP), Type 2 patients requiring careful subtype-specific approaches, and Type 3 patients necessitating regular prophylactic factor replacement therapy to prevent serious bleeding episodes.
Age Distribution of Von Willebrand Disease Patients in US 2025
| Age Group | Type 1 VWD | Type 2 VWD | Type 3 VWD | Total Patients | Percentage of Total |
|---|---|---|---|---|---|
| Under 2 years | 236 | 61 | 10 | 391 | 1.1% |
| 2-10 years | 4,819 | 631 | 65 | 6,216 | 17.5% |
| 11-19 years | 11,798 | 1,008 | 89 | 14,131 | 39.8% |
| 20-44 years | 7,931 | 1,218 | 229 | 10,310 | 29.1% |
| 45-64 years | 1,980 | 497 | 90 | 2,883 | 8.1% |
| 65+ years | 877 | 393 | 48 | 1,535 | 4.3% |
| Total | 27,641 | 3,808 | 531 | 35,466 | 100% |
Data Source: Centers for Disease Control and Prevention (CDC) Community Counts Program, HTC Population Profile Data from January 1, 2012 through March 31, 2024
The age distribution of Von Willebrand Disease patients in the US 2025 reveals distinct patterns reflecting both disease presentation and healthcare utilization across different life stages. The largest patient cohort falls within the 11-19 age range with 14,131 patients representing nearly 40% of all tracked VWD cases. This concentration reflects the critical period when adolescents and teenagers experience symptoms that prompt diagnosis, particularly among females beginning menstruation who may experience heavy menstrual bleeding. Within this age group, Type 1 VWD accounts for 11,798 patients, while Type 2 comprises 1,008 patients and Type 3 includes 89 patients, demonstrating that the adolescent years represent a peak diagnostic and treatment period across all disease severities.
The second-largest age cohort encompasses 20-44 years with 10,310 patients or 29% of the total population, representing the prime reproductive years when women with VWD face additional challenges related to pregnancy, childbirth, and ongoing menstrual management. The pediatric population aged 2-10 years comprises 6,216 patients representing 17.5% of cases, with diagnoses often occurring when children experience abnormal bleeding following minor injuries, dental procedures, or surgical interventions. Notably, Type 3 VWD patients show relatively higher representation in the 20-44 age group with 229 patients compared to younger age groups, suggesting that individuals with severe disease who survive childhood continue to require intensive specialized care throughout adulthood. The elderly population aged 65 years and older represents 1,535 patients or 4.3%, a relatively smaller proportion that may reflect both historical underdiagnosis and potential survival challenges for those with more severe forms. The youngest category, children under 2 years, accounts for only 391 patients or 1.1%, as VWD diagnosis in infancy remains relatively uncommon except in severe Type 3 cases where bleeding manifestations may be evident from birth.
Gender Distribution of Von Willebrand Disease in US 2025
| VWD Type | Male Patients | Male % | Female Patients | Female % | Total |
|---|---|---|---|---|---|
| Type 1 | 8,881 | 32% | 18,760 | 68% | 27,641 |
| Type 2 | 1,538 | 40% | 2,270 | 60% | 3,808 |
| Type 3 | 258 | 49% | 273 | 51% | 531 |
| Type Other/Unknown | 1,067 | 31% | 2,419 | 69% | 3,486 |
| All Types Combined | 11,744 | 33% | 23,722 | 67% | 35,466 |
Data Source: CDC Community Counts, Von Willebrand Disease Patient Characteristics, January 1, 2012 – March 31, 2024
The gender distribution of Von Willebrand Disease patients receiving care at hemophilia treatment centers demonstrates significant variation across different disease types, providing important insights into symptom presentation and healthcare-seeking behavior. Overall, female patients comprise 67% of the total VWD population with 23,722 patients, while male patients represent 33% with 11,744 patients. This pronounced disparity is most evident in Type 1 VWD, where women account for 68% with 18,760 patients compared to 32% or 8,881 male patients. The gender imbalance in Type 1 disease reflects the reality that women are significantly more likely to experience noticeable symptoms requiring medical attention, particularly heavy menstrual bleeding affecting up to 74-92% of women with diagnosed VWD.
Interestingly, the gender distribution becomes more balanced in more severe forms of the disease. Type 3 VWD shows nearly equal representation with 49% male patients (258 individuals) and 51% female patients (273 individuals), suggesting that the severe bleeding manifestations of complete VWF absence are equally apparent and diagnostically pursued regardless of gender. Type 2 VWD demonstrates an intermediate pattern with 60% female and 40% male patients, indicating that while women still predominate, the qualitative defects in VWF function produce bleeding symptoms notable enough in men to prompt diagnosis and treatment more frequently than the milder Type 1 disease. This gender distribution pattern underscores a critical aspect of Von Willebrand Disease epidemiology in 2025: genetic inheritance affects males and females equally, but clinical presentation, symptom recognition, and healthcare utilization differ substantially based on biological factors, particularly the impact of menstruation, pregnancy, and childbirth on bleeding manifestations in women.
Racial and Ethnic Distribution of Von Willebrand Disease in US 2025
| Race/Ethnicity | Type 1 VWD | Type 1 % | Type 2 VWD | Type 2 % | Type 3 VWD | Type 3 % |
|---|---|---|---|---|---|---|
| White | 22,562 | 82% | 3,045 | 80% | 410 | 77% |
| Black/African American | 1,985 | 7% | 347 | 9% | 47 | 9% |
| Asian | 712 | 3% | 115 | 3% | 39 | 7% |
| American Indian/Alaska Native | 184 | 1% | 28 | 1% | 6 | 1% |
| Native Hawaiian/Pacific Islander | 93 | 0.3% | 13 | 0.3% | <5 | <1% |
| More than One Race | 311 | 1% | 49 | 1% | <5 | <1% |
| Unknown | 1,794 | 6% | 211 | 6% | 21 | 4% |
| Hispanic/Latino (Ethnicity) | 4,928 | 18% | 571 | 15% | 53 | 10% |
Data Source: CDC Community Counts Program, Von Willebrand Disease Patient Demographics, January 2012 – March 2024
The racial and ethnic composition of Von Willebrand Disease patients in the United States reflects complex interactions between genetic factors, healthcare access patterns, and demographic population distributions across the country. White patients constitute the overwhelming majority across all VWD types, representing 82% of Type 1 patients (22,562 individuals), 80% of Type 2 patients (3,045 individuals), and 77% of Type 3 patients (410 individuals). This predominance reflects both the demographic composition of the U.S. population and the historical development of hemophilia treatment centers in areas with higher concentrations of White populations, though it may also indicate disparities in diagnosis, referral patterns, and access to specialized bleeding disorder care.
Black and African American patients comprise the second-largest racial group, accounting for 7% of Type 1 VWD cases (1,985 patients) and 9% each of Type 2 and Type 3 cases (347 and 47 patients respectively). The slightly higher representation in more severe disease types may suggest that severe bleeding manifestations are more likely to result in diagnosis regardless of potential healthcare access barriers. Asian patients represent 3% of Type 1 and Type 2 cases but notably constitute 7% of Type 3 VWD patients (39 individuals), a proportion that exceeds their representation in milder forms. Hispanic or Latino patients, tracked separately as an ethnicity rather than race, comprise 18% of Type 1 VWD patients (4,928 individuals), 15% of Type 2 patients (571 individuals), and 10% of Type 3 patients (53 individuals). This substantial Hispanic representation reflects the growing Latino population in the United States and improving access to specialized hemophilia treatment centers in communities with significant Hispanic populations. The relatively small percentages of American Indian/Alaska Native patients (1% across all types) and Native Hawaiian/Pacific Islander patients (less than 1%) may indicate both smaller population sizes and potential challenges in accessing federally funded hemophilia treatment centers located primarily in urban areas distant from tribal lands and Pacific Islander communities.
Von Willebrand Disease Treatment Patterns in US 2025
| Treatment Approach | Applicable VWD Types | Usage Rate | Mechanism of Action |
|---|---|---|---|
| Desmopressin (DDAVP) | Type 1, Some Type 2 | Primary for 60-80% Type 1 cases | Releases stored VWF from endothelial cells |
| Factor Replacement Therapy | All types, especially Type 3 | Essential for Type 3, backup for others | Provides exogenous VWF and Factor VIII |
| Recombinant VWF Products | Type 3, Severe Type 2 | Increasing adoption since 2015 | Plasma-free VWF replacement |
| Birth Control Pills | Type 1 and 2 (women) | High usage for menstrual bleeding | Increases VWF and Factor VIII levels |
| Antifibrinolytic Agents | All types | Adjunctive for minor procedures | Prevents clot breakdown |
| Prophylactic Treatment | Severe Type 3 | Routine for severe cases | Prevents spontaneous bleeding |
| On-Demand Treatment | Type 1 and 2 | Most common approach | Treats bleeding episodes as they occur |
Data Source: National Institutes of Health VWD Guidelines 2021, FDA Approved Treatments 2015-2024, CDC Treatment Center Reports
Treatment strategies for Von Willebrand Disease in 2025 have evolved significantly with expanded therapeutic options enabling more personalized and effective management across all disease types. Desmopressin (DDAVP) remains the first-line treatment for the majority of Type 1 VWD patients, effectively managing 60-80% of cases by stimulating the release of von Willebrand factor stored in blood vessel linings. This synthetic hormone, administered via injection, intravenous infusion, or nasal spray, offers a convenient option for managing minor bleeding episodes and providing prophylaxis before dental procedures or minor surgeries. However, DDAVP effectiveness varies considerably among patients, requiring trial doses to determine individual responsiveness before relying on this treatment for procedural management or acute bleeding situations.
Factor replacement therapy utilizing plasma-derived VWF/Factor VIII concentrates represents the cornerstone treatment for Type 3 VWD patients who have complete absence of von Willebrand factor and cannot respond to DDAVP. The FDA approval of recombinant von Willebrand factor (Vonvendi) in 2015 marked a significant advancement, providing a plasma-free alternative that eliminates viral transmission risks while demonstrating excellent bleeding control in 96.9% of treated episodes across minor, moderate, and major bleeding events. Expanded FDA approvals in 2018 for perioperative management and in 2022 for routine prophylaxis in severe Type 3 VWD patients reflect growing recognition that proactive prevention strategies, rather than reactive on-demand treatment, improve long-term outcomes including musculoskeletal health and quality of life. Hormonal contraceptives serve dual purposes for women with VWD, simultaneously preventing pregnancy while increasing von Willebrand factor and Factor VIII levels to reduce menstrual blood loss, representing a practical therapeutic approach for the 68% female patient population experiencing heavy menstrual bleeding. Antifibrinolytic medications including tranexamic acid and aminocaproic acid provide valuable adjunctive therapy by preventing the premature breakdown of blood clots, particularly useful for dental procedures, minor surgeries, and managing mucosal bleeding where these agents can be applied topically or taken orally to enhance hemostasis without systemic factor replacement.
Von Willebrand Disease Healthcare Access in US 2025
| Healthcare Metric | Type 1 VWD | Type 2 VWD | Type 3 VWD | Overall Impact |
|---|---|---|---|---|
| Insurance Coverage | 98% insured | 93% insured | 98% insured | High access to care |
| Federal HTC Network | 140+ centers nationwide | Comprehensive coverage | Specialized services | Geographic accessibility |
| Uninsured Rate | 1% (361 patients) | 6% (214 patients) | <1% | Minimal barriers to coverage |
| Patients Tracked 2012-2024 | 27,641 | 3,808 | 531 | 35,466 total |
| Multidisciplinary Teams | Available at all HTCs | Hematologists, nurses, social workers | Comprehensive approach | Holistic patient care |
| Home Treatment Programs | Widely available | Self-infusion training | Emergency protocols | Patient independence |
Data Source: CDC Community Counts, U.S. Hemophilia Treatment Center Network Statistics 2024
Healthcare access for Von Willebrand Disease patients in the United States demonstrates remarkably high insurance coverage rates, with 98% of Type 1 VWD patients having insurance, 93% of Type 2 patients insured, and 98% of Type 3 patients covered. These high coverage rates facilitate access to the nationwide network of over 140 federally funded hemophilia treatment centers providing specialized comprehensive care for bleeding disorders. The federal HTC network, established in 1975 to ensure access to specialized care, offers multidisciplinary teams including hematologists, nurses, social workers, physical therapists, and mental health professionals who collaborate to address the complex medical and psychosocial needs of VWD patients throughout their lifespan.
The extremely low uninsured rates—only 1% among Type 1 patients (361 individuals) and less than 1% among Type 3 patients—represent a stark contrast to the general U.S. population where uninsured rates remain significantly higher. This favorable insurance coverage pattern likely reflects several factors including the recognition of VWD as a chronic condition qualifying for comprehensive coverage, the availability of patient assistance programs through pharmaceutical manufacturers and nonprofit organizations, and the advocacy efforts of the bleeding disorders community to ensure access to expensive factor replacement therapies. Geographic accessibility to specialized care varies across states, with HTC-treated prevalence ranging from 0.7 cases per 100,000 in Delaware to 22.7 cases per 100,000 in Connecticut, suggesting that proximity to treatment centers and regional awareness among healthcare providers influence diagnosis and referral patterns. Home treatment programs enable patients, particularly those with Type 3 VWD requiring regular prophylaxis, to achieve greater independence by learning self-infusion techniques and managing bleeding episodes promptly without emergency department visits. The CDC’s Community Counts surveillance program, tracking 35,466 patients from 2012 through March 2024, provides the infrastructure for ongoing monitoring of health outcomes, complications, and mortality, ensuring data-driven improvements in VWD care delivery across the United States.
Von Willebrand Disease Complications and Bleeding Manifestations in US 2025
| Bleeding Type | Frequency in VWD Population | Severity by Type | Management Approach |
|---|---|---|---|
| Heavy Menstrual Bleeding | 74-92% of women | Type 1-2 primarily | Hormonal therapy, DDAVP, factor replacement |
| Nosebleeds (Epistaxis) | 38-63% of patients | All types | Local pressure, antifibrinolytics, cauterization |
| Easy Bruising | 36% of patients | More common in Type 1-2 | Education, activity modification |
| Dental Procedure Bleeding | 29-52% of patients | All types | Prophylactic treatment before procedures |
| Post-Surgical Bleeding | 20-28% of patients | Risk increases with severity | Pre-operative factor replacement |
| Gastrointestinal Bleeding | 4-24% of patients | More common in older adults | Endoscopic intervention, factor support |
| Joint Bleeding (Hemarthrosis) | 6-8% of patients | Type 3 primarily | Prophylaxis, orthopedic management |
| Wound-Related Bleeding | 38-79% of patients | Post-trauma or surgical | Topical hemostatics, factor replacement |
Data Source: Von Willebrand Disease Epidemiology Systematic Review 2010-2021, CDC Clinical Characteristics Data, ASH/ISTH/NHF/WFH Guidelines 2021
Bleeding manifestations in Von Willebrand Disease vary considerably based on disease type, severity, and individual patient factors, with mucocutaneous bleeding representing the hallmark clinical feature across all VWD types. Heavy menstrual bleeding affects 74-92% of women diagnosed with VWD, making it the single most common symptom prompting diagnosis and a primary driver of the 68% female predominance in diagnosed cases. This severe menstrual blood loss often necessitates changing pads or tampons more than once per hour, periods lasting longer than seven days, and frequently results in iron-deficiency anemia requiring supplemental treatment. Epistaxis, or nosebleeds, occurs in 38-63% of VWD patients, with episodes typically lasting longer than 10 minutes and sometimes requiring medical intervention including nasal packing, cauterization, or factor replacement when standard local pressure measures fail.
Post-surgical and procedural bleeding presents significant challenges, affecting 20-28% of patients undergoing operations and 29-52% experiencing prolonged bleeding after dental extractions. These statistics underscore the critical importance of pre-procedural hematology consultation and prophylactic treatment with either DDAVP or factor replacement products to maintain adequate hemostasis throughout the perioperative period and healing phase. Gastrointestinal bleeding, reported in 4-24% of the VWD population, occurs more frequently in older adults with the highest rates observed in patients aged 65-85 years, potentially reflecting both acquired vascular abnormalities and the cumulative effects of chronic VWD on the gastrointestinal mucosa. Joint bleeding and hemarthrosis, traditionally associated with severe hemophilia, affects 6-8% of VWD patients with rates reaching 38% in some severe Type 3 patient cohorts, necessitating orthopedic evaluation, physical therapy, and prophylactic factor replacement to prevent chronic joint damage. The broad range of bleeding manifestations, from minor bruising affecting 36% of patients to life-threatening hemorrhages in severe Type 3 disease, emphasizes the importance of individualized treatment plans, patient education regarding bleeding prevention strategies, and ready access to appropriate hemostatic therapies when bleeding episodes occur.
Von Willebrand Disease Mortality and Long-Term Outcomes in US 2025
| Health Outcome | VWD vs General Population | Specific Findings | Implications |
|---|---|---|---|
| All-Cause Mortality | No significant difference | Hazard ratio 0.90 (95% CI 0.80-1.05) | VWD not associated with increased death risk |
| Life Expectancy | Comparable to general population | Normal lifespan with proper management | Effective modern treatment protocols |
| Quality of Life Impact | Lower HRQoL scores vs general population | Bleeding impacts daily activities | Need for comprehensive support services |
| Anemia Prevalence | High in women with VWD | Iron deficiency from chronic blood loss | Requires iron supplementation monitoring |
| Joint Health | Type 3 patients at risk | Hemarthrosis can cause chronic damage | Prophylaxis prevents long-term complications |
| Pregnancy Outcomes | Increased bleeding risk | Higher postpartum hemorrhage rates | Requires specialized obstetric management |
| Healthcare Utilization | Increased vs general population | More frequent medical visits and interventions | Substantial healthcare system burden |
Data Source: Swedish Cohort Study 2790 Patients 1987-2009, U.S. Single-Center Study 198 Patients 1985-2010, VWD Systematic Review 2023
Long-term outcome data for Von Willebrand Disease patients provide reassuring evidence that with appropriate diagnosis and management, individuals with VWD can expect normal lifespans comparable to the general population. A large Swedish cohort study following 2,790 VWD patients over a 22-year observation period from 1987-2009 found all-cause mortality of 10.8%, equivalent to 235 deaths per 100,000 person-years, with the hazard ratio of 0.90 (95% confidence interval 0.80-1.05) indicating no statistically significant difference in death risk compared to age and sex-matched individuals without VWD. Similarly, a U.S. single-center cohort study comparing 198 VWD patients to 198 matched controls found mortality rates of 5.1% versus 2.0% respectively, a difference that did not reach statistical significance.
While mortality rates remain reassuringly normal, quality of life measures reveal that VWD patients experience lower health-related quality of life scores compared to general populations, primarily driven by the chronic burden of bleeding symptoms, activity restrictions, anxiety about spontaneous bleeding episodes, and the psychological impact of living with an inherited bleeding disorder. Women with VWD face particular challenges related to heavy menstrual bleeding throughout their reproductive years, frequently developing iron-deficiency anemia requiring ongoing supplementation and monitoring, and experiencing social limitations during menstruation. Pregnancy and childbirth present elevated risks with increased rates of postpartum hemorrhage, necessitating specialized obstetric care, close coordination between hematology and obstetrics teams, and sometimes prophylactic factor replacement during labor and the immediate postpartum period. Healthcare resource utilization among VWD patients significantly exceeds that of the general population, with increased frequency of medical visits, emergency department presentations for bleeding episodes, surgical interventions, and need for specialized diagnostic testing and monitoring. However, patients receiving care within the federally funded hemophilia treatment center network benefit from comprehensive multidisciplinary management addressing not only bleeding complications but also psychosocial support, physical therapy for joint health, genetic counseling for family planning, and coordination with community healthcare providers, substantially improving overall outcomes and enabling most VWD patients to lead full, productive lives despite their bleeding disorder.
Disclaimer: The data research report we present here is based on information found from various sources. We are not liable for any financial loss, errors, or damages of any kind that may result from the use of the information herein. We acknowledge that though we try to report accurately, we cannot verify the absolute facts of everything that has been represented.